Serology tests detect the presence of specific proteins of interest in the serum (the liquid phase of blood after clotting).
Serology tests for COVID-19 aim to detect in the serum specific antibodies against SARS-CoV-2 (antibodies are proteins as well).
The human body produces 5 different types of antibodies (IgA, IgD, IgE, IgG e IgM, where “Ig” stand for immunoglobulins,another word for antibodies), but in the case of SARS-CoV-2 infection, only IgG and IgM are of interest.
IgM are produced in the initial phases of infection (usually during the first week) and circulate for a limited time. IgG are produced about 10-12 days after the beginning of the infection, but they remain in circulating for longer (how long depends on the specific pathogen). During a certain amount of time, both IgM and IgG can be present in the serum of an individual.
The presence of IgM indicates a very recent or still present infection, while the presence of IgG only indicates a past infection.
Among serology tests, we can distinguish between quantitative tests, able to determine the concentration of the antibodies of interest, and qualitative tests, that determine the presence or absence of the antibodies.
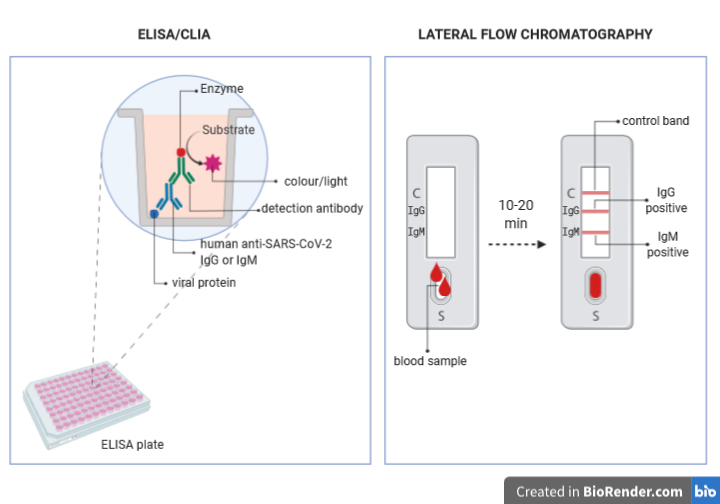
Hospitals and authorised laboratories perform quantitative tests: ELISA (enzyme-linked immunosorbent assay ) and CLIA (chemioluminescence assay).
The principle of these techniques is as follows:
- Viral proteins are stably linked on a solid support (the bottom of a plate well)
- The serum to be tested is added to the wells in different dilutions; if the serum contains specific antibodies, they will bind to the viral proteins
- An antibody able to recognize human IgG or IgM (detection antibody) is added to the well; if the patient serum contained anti-SARS-CoV-2 antibodies, the detection antibodies will bind them. The detection antibodies are modified so that they are bound (conjugated) to a molecule (enzyme) able to produce colour (in the case of ELISA) or light (in the case of CLIA) when a specific molecule (substrate) is added to the sample.
- The appropriate substrate is added to the wells and the intensity of the colour or of the light produced by the reaction with the enzyme conjugated to the detection antibody, and its intensity is measured.
- Based on the intensity of the colour or light, the concentration of the anti-SARS-CoV-2 antibodies present in the patient serum is calculated.
The rapid tests that have become so popular in the last weeks are qualitative tests based on a technology named Lateral Flow Chromatography.
The device to perform these tests are little strips of a special resin, where ant-IgM and anti-IgG are placed in different points.
- A drop of blood to be tested is placed in a well at one extremity of the device.
- The liquid phase of the blood (containing the antibodies) will flow by capillarity through the strip and it will go across the conjugation chamber, where it will encounter viral proteins conjugated to colloidal gold (pink-red coloured).
- If the patient blood contains antibodies against the viral proteins, they will bind them and they will carry them while flowing in the strip until they encounter the anti-IgG or anti-IgM bound to the device.
- The binding of the anti-antibodies to the IgG or IgM bound to the viral proteins conjugated to the colloidal gold particles, will determine the appearance of a red band, revealing the presence or absence of the antibodies ion the sample.
- The intensity of the band can indicate whether there is a small or big amount of antibodies in the sample, but it will not be possible to calculate their exact concentration.
These devices have also a control system, that is a third band to which antibodies that recognize non-human antibodies against other proteins are bound. Those non-human antibodies and their target proteins are placed in the conjugation chamber and will move by capillarity through the strip together with the sample until they reach their anti-antibodies. Only if the control band turns red, the test will be considered valid.
(These are just two examples of the many techniques in which antibodies are used as tools in the lab.)
Images created in BioRender.com
Bibliography:
Immunobiology: The Immune System in Health and Disease. 5th edition.The distribution and functions of immunoglobulin isotypes, Janeway C.A. Jr et al., New York: Garland Science 2001https://www.ncbi.nlm.nih.gov/books/NBK27162/
Chemiluminescent immunoassay technology: what does it changein autoantibody detection?, Cinquanta L. et al., Autoimmun Highlights 2017 https://doi.org/10.1007/s13317-017-0097-2
Enzyme-linked immunosorbent assay (ELISA): the basics,Shah K. et al., British Journal of Hospital Medicine 2016 https://doi.org/10.12968/hmed.2016.77.7.C98
Lateral Flow Technology for Field-Based Applications—Basics and Advanced Developments,O’Farrell B., Topics in Compan An Med 2015 https://doi.org/10.1053/j.tcam.2015.12.003